- PMID: 15061632
- PMCID: PMC387438
Abstract
Coronary artery bypass grafting prolongs survival in patients with left main coronary artery stenosis. However, this benefit is denied to patients who refuse the procedure or who are poor surgical candidates due to comorbid conditions. We describe a novel technique for the percutaneous revascularization of stenosis in an unprotected left main coronary artery in high-risk patients. The TandemHeart, a percutaneously inserted left ventricular assist device, was used to provide periprocedural hemodynamic support during angioplasty and stenting of an unprotected left main coronary artery for stenosis in a 70-year-old woman. The device was removed immediately after the procedure, and the patient was discharged from the hospital on the 2nd postprocedural day. The potential advantages of angioplasty with the support of percutaneous left ventricular assist devices in high-risk patients are discussed.
Keywords: Angioplasty, transluminal, percutaneous coronary/methods; hemodynamic support; coronary disease/mortality; coronary stenosis/therapy; coronary vessels/pathology; heart assist devices; myocardial revascularization; stents
Coronary artery bypass grafting (CABG) is the treatment of choice for coronary artery disease in an unprotected left main coronary artery; however, the risk of perioperative morbidity and death can be unacceptably high in the presence of comorbid conditions. In such cases, percutaneous transluminal coronary angioplasty (PTCA) and stenting can produce good short- and long-term results but may be associated with high mortality, particularly in patients whose left ventricular function is poor. 1–3 This increased risk is due, in part, to the detrimental hemodynamic effects that can occur during high-risk percutaneous interventions. A number of circulatory support techniques have been used to avoid this complication, including intra-aortic balloon counterpulsation (IABP) 4,5 and extracorporeal membrane oxygenation. 6 In the present patient, we describe the use of a percutaneous left ventricular assist device (pVAD) to provide effective hemodynamic support during stenting of an unprotected left main coronary artery.
Case Report
In September 2003, a 70-year-old woman presented at our institution with unstable angina. Her relevant medical history included coronary artery disease and an ascending aortic aneurysm that had been treated 8 months earlier by CABG and aortic arch reconstruction. Revascularization consisted of a saphenous vein graft to the left anterior descending coronary artery, a saphenous vein graft to the posterior descending coronary artery, and a left internal mammary artery graft to the ramus intermedius. After the operation, the patient had a protracted, complicated rehabilitation period that lasted for 6 months. In addition, her medical history included peripheral vascular disease, hypertension, hypercholesterolemia, breast carcinoma that had been treated with radiation, and cholelithiasis. The patient was currently receiving maximal medical therapy for angina.
At the September presentation, coronary angiography revealed 90% to 95% stenosis of the ostium of the left main coronary artery; total occlusion of the proximal right coronary artery, the saphenous vein graft to the left anterior descending coronary artery, and the left internal mammary artery graft to the ramus intermedius; and patency of the saphenous vein graft to the posterior descending artery. The patient’s left ventricular ejection fraction was 0.55 to 0.60. The patient was not a candidate for repeat surgical revascularization, because 1) her cardiothoracic anatomy had been distorted both by the radiation therapy for her breast cancer and by her first CABG surgery and 2) she was unwilling to undergo a 2nd CABG procedure. Instead, PTCA of the unprotected left main coronary artery was chosen.
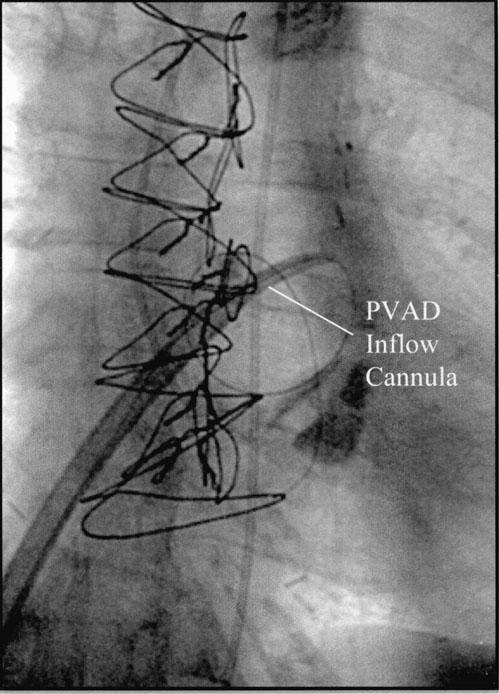
Periprocedural hemodynamic support was provided by an axial-flow pVAD (TandemHeart™, CardiacAssist, Inc.; Pittsburgh, Pa). 7 Because pre-existing peripheral vascular disease in the patient’s left common iliac artery would have prevented placement of the pVAD’s outflow cannula, the left common iliac artery first had to be revascularized with use of percutaneous transluminal angioplasty and implantation of an 8.0- × 27-mm Express™ stent (Boston Scientific Corporation; Boston, Mass). After this was accomplished, the outflow cannula of the pVAD was inserted over a guidewire into the left iliac artery. A transseptal puncture was then performed, and the pVAD inflow cannula was advanced under direct fluoroscopic guidance into the left atrium (Fig. 1). After hemodynamic support was established, PTCA was performed, along with stenting of the left main coronary artery with a Cypher™ sirolimus-eluting stent (Cordis, Inc.; Miami Lakes, Fla). Although the patient’s systolic blood pressure dropped to less than 90 mmHg during balloon inflation, the pVAD was able to maintain cardiac output at 3 to 4 L/min. Intraoperative angiography confirmed successful revascularization of the artery (Fig. 2).
The patient’s chest pain resolved completely after coronary revascularization, and she was weaned from pVAD support immediately after the procedure. The pVAD cannulae were removed, and the left femoral artery was repaired surgically in the cardiac catheterization laboratory under local anesthesia. The patient’s subsequent postoperative course was uneventful, and she was discharged from the hospital in good condition on the 2nd postoperative day. When last seen in mid-January 2004, the patient was doing well.
Discussion
Percutaneous revascularization of an unprotected left main coronary artery is a high-risk procedure that can be life-saving in patients who are not candidates for CABG. Hemodynamic support during such a procedure, however, must maintain coronary perfusion at a level sufficient to preserve myocardial function, reduce complications, and minimize procedure-related deaths. Intra-aortic balloon counterpulsation is the most commonly used method of hemodynamic support during high-risk percutaneous interventions, including those in the left main coronary artery. 5 As the present case demonstrates, however, the TandemHeart pVAD may be a feasible alternative to IABP. Moreover, early experience with this device 7 has shown that the TandemHeart may be more advantageous. First, the blood flow provided by the TandemHeart is sufficient to maintain both coronary and peripheral perfusion. Second, and more important, the TandemHeart can provide adequate circulatory support regardless of the native heart rhythm. Third, the TandemHeart can be used for up to 2 weeks while producing relatively few thromboembolic and hemorrhagic complications. 7
Thus far, nearly all of the preliminary experience with the TandemHeart pVAD in high-risk percutaneous interventions has been in patients without left main coronary artery disease. 7 The present case report extends this experience to a high-risk procedure in a patient with coronary artery disease in an unprotected left main coronary artery. In all high-risk patients, however, balloon inflation can cause ischemia in a large area of myocardium, which can, in turn, lead to asystole or ventricular fibrillation. The pVADs may prove to be particularly useful in such situations: by providing adequate hemodynamic support during prolonged periods of asystole, the device could theo-retically allow the operator more time to complete a high-risk procedure or it could serve as a bridge to surgical revascularization. This potential use of the pVAD for high-risk coronary interventions, particularly in patients with coronary artery disease in an unprotected left main coronary artery, warrants further study.
Footnotes
Address for reprints: Biswajit Kar, MD, 6720 Bertner Avenue, C355M, Houston, TX 77030
E-mail: moc.loa@6rakb
References
Articles from Texas Heart Institute Journal are provided here courtesy of Texas Heart Institute
Hemodynamic support with a percutaneous approach is a vital medical procedure used to stabilize patients with severe circulatory or heart conditions. This technique involves the use of a minimally invasive catheter-based system to provide circulatory assistance, ensuring adequate blood flow to vital organs when the heart is unable to pump effectively. This method is often used in patients experiencing acute heart failure, cardiogenic shock, or during complex cardiac surgeries.
The primary goal of hemodynamic support with a percutaneous device is to enhance cardiac output and support the heart in pumping blood, especially when the body’s natural circulatory function is compromised. Devices such as intra-aortic balloon pumps (IABP) or percutaneous left ventricular assist devices (LVAD) are commonly employed in these procedures. These devices help restore blood flow to critical areas, such as the brain, kidneys, and heart, which can otherwise be jeopardized in severe cases of circulatory failure.
A key benefit of hemodynamic support with a percutaneous technique is that it is less invasive compared to traditional open-heart surgery. By inserting a catheter through a small incision, doctors can deliver life-saving support without the need for large surgical cuts, reducing patient trauma, speeding up recovery, and minimizing the risk of infection. This approach is particularly beneficial for critically ill patients who are not candidates for open-heart surgery or those who need immediate circulatory assistance.
Overall, hemodynamic support with a percutaneous approach represents a significant advancement in the treatment of acute cardiovascular conditions. It provides vital support for patients in life-threatening situations, helping them stabilize and recover, while offering a less invasive and safer alternative to traditional surgical interventions.